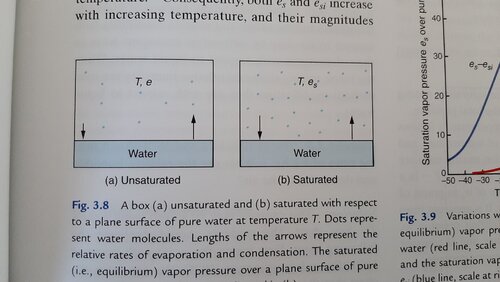
I watched a lecture that was explaining Saturation Vapor Pressure of water. I understand that saturation occurs when the amount of water evaporating off the surface of a water source is exactly equal to the amount of water that is condensing out of the air above (one molecule goes from liquid to vapor while another molecule goes from vapor to liquid).
Along with this, the concept of Partial Pressure is also needed in order to understand what’s happening.
Partial Pressure is the portion of the atmospheric pressure that is attributed to a given substance in the air.
Atmospheric pressure is the simple addition of all the Partial Pressures being created by all of the atmosphere’s contents. Therefore, since 78% of the atmosphere is Nitrogen, 78% of atmospheric pressure is due to the pressure being created by Nitrogen. If all the Nitrogen magically disappeared instantly, the atmospheric pressure would drop by 78%.
So far, everything matches my intuition.
But then, the professor said that the Partial Pressure of water alone determines when saturation occurs, and that “it actually doesn’t matter whether there is air above the liquid water or not because Saturation Vapor pressure is a property of the water, not of the air.
“It’s a mistake to to say things like, the air is saturated. That’s not quite right, because it’s the water vapor we’re talking about.
“Now, it’s interesting that that water is mixed into the air, but the air is not really controlling this. This is the property of water alone.
“You’d have the same Partial Pressure of water vapor above a liquid at a certain temperature whether there was air there or not.
“Of course, if there was air there, the total pressure would be higher, but the Partial Pressure of water vapor would be the same value.”
It sounds like to me that the professor is saying that the evaporation of water doesn’t care about all the other things that are exerting pressure on it, that somehow the evaporating water is only being affected by the pressure in the air that’s being created by other water molecules.
What is the physics behind this? I don’t understand that if evaporation is affected by pressure, then why would only certain pressures count rather than all the pressures that are present?
Along with this, the concept of Partial Pressure is also needed in order to understand what’s happening.
Partial Pressure is the portion of the atmospheric pressure that is attributed to a given substance in the air.
Atmospheric pressure is the simple addition of all the Partial Pressures being created by all of the atmosphere’s contents. Therefore, since 78% of the atmosphere is Nitrogen, 78% of atmospheric pressure is due to the pressure being created by Nitrogen. If all the Nitrogen magically disappeared instantly, the atmospheric pressure would drop by 78%.
So far, everything matches my intuition.
But then, the professor said that the Partial Pressure of water alone determines when saturation occurs, and that “it actually doesn’t matter whether there is air above the liquid water or not because Saturation Vapor pressure is a property of the water, not of the air.
“It’s a mistake to to say things like, the air is saturated. That’s not quite right, because it’s the water vapor we’re talking about.
“Now, it’s interesting that that water is mixed into the air, but the air is not really controlling this. This is the property of water alone.
“You’d have the same Partial Pressure of water vapor above a liquid at a certain temperature whether there was air there or not.
“Of course, if there was air there, the total pressure would be higher, but the Partial Pressure of water vapor would be the same value.”
It sounds like to me that the professor is saying that the evaporation of water doesn’t care about all the other things that are exerting pressure on it, that somehow the evaporating water is only being affected by the pressure in the air that’s being created by other water molecules.
What is the physics behind this? I don’t understand that if evaporation is affected by pressure, then why would only certain pressures count rather than all the pressures that are present?